Covid-19 Vaccine: Johnson & Johnson expedite human trials, India’s Panacea Biotec partners with US firm
Coronavirus (Covid-19) Vaccine Latest Update: Among Covid-19 vaccine front runners in human trials are AstraZeneca, Pfizer, BioNtech, Johnson & Johnson, Merck, Moderna, Sanofi and China’s CanSino Biologics
Even though the US and China seem to be on the global frontline in terms of developing a Covid-19 vaccine, an Italian Health Ministry official said Europe was far ahead in the race and the results of ongoing research could lead to first doses “by autumn-winter”.
Meanwhile, Johnson & Johnson (J&J) has decided to expedite the start of human clinical trials for its experimental Covid-19 vaccine by two months to the second half of July. The move saw its shares rising nearly 2 per cent to $148.69. On the other hand, Moderna Inc has started testing its vaccine candidate in a 600-subject mid-stage trial.
Coming to India, New Delhi-based biotechnology company Panacea Biotec has partnered with US-based Refana Inc to develop, manufacture and distribute an experimental Covid-19 vaccine, Reuters reported.
More than 100 potential Covid-19 vaccines are in various stages of development around the world. Among front runners currently in human trials are the Covid-19 vaccines being developed by AstraZeneca, Pfizer, BioNtech, Johnson & Johnson, Merck, Moderna, Sanofi, and China’s CanSino Biologics.
Coronavirus (Covid-19) vaccine latest updates:
💉 Initially planned for September, American firm Johnson & Johnson said it had fast-tracked the start of human clinical trials for its recombinant Ad26.COV2-S vaccine by two months to the second half of July. The decision may allow J&J to take part in the massive clinical trials program — Operation Warp Speed — planned by the US government.
In March, J&J signed deals with the US government to create enough manufacturing capacity to produce more than 1 billion doses of its vaccine through 2021, Reuters reported.
“Based on the strength of the pre-clinical data we have seen so far and interactions with the regulatory authorities, we have been able to further accelerate the clinical development,” said Chief Scientific Officer Paul Stoffels.
The study will test the vaccine for safety and early signs of efficacy in 1,045 healthy volunteers aged 18 to 55 years, and in those aged 65 and older. The trial will take place in the United States and Belgium.
The company is also in talks with the National Institutes of Allergy and Infectious Diseases(NIAID) to start larger, late-stage trials ahead of schedule.
For developing the Covid-19 vaccine, the technology being applied is similar to the one used to develop their investigational Ebola vaccine, as well as vaccine candidates for the Zika virus, RSV and HIV.
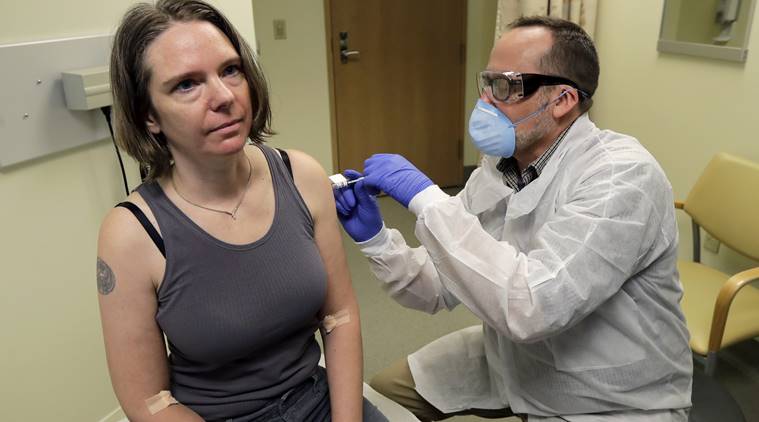 A pharmacist gives a woman the first shot in the first-stage safety study clinical trial of a potential vaccine for COVID-19. (AP)
A pharmacist gives a woman the first shot in the first-stage safety study clinical trial of a potential vaccine for COVID-19. (AP)
💉 Coming to India, Panacea Biotec has become the fifth biotech company from the country to join global efforts in developing a Covid-19 vaccine by partnering with US-based Refana Inc.
In a statement to the stock exchanges, Panacea said the collaboration aims to make more than 500 million doses
of the inactivated virus-based vaccine, with over 40 million doses expected to be available early next year. The firm said a joint venture firm would be set up in Ireland.
As per the partnership, Panacea Biotec will be responsible for product development and commercial manufacturing, while the JV entity undertaking clinical development and regulatory submissions across the world. Both Panacea and Refana will undertake sales and distribution of the vaccine in their respective territories.
“Whole inactivated viral vaccines have a higher probability of being safe and efficacious, given their long history and better understanding of their mechanism of action, which has been elucidated over many decades,” PTI quoted Panacea Biotec Managing Director Rajesh Jain as saying.
While the US and China may have grabbed the headlines on vaccine research and development, Europe seems to be not far behind. The adviser to the Italian Health Ministry for Covid-19 emergency told public TV RAI 3 that a vaccine research project conducted by an Anglo-Italian partnership was “in an advanced development phase”.
“Europe is far ahead of the United States in terms of new coronavirus vaccine, and we are getting ready for having a consistent part of it produced in Italy. With respect to the timing, if all goes well, we might have the first doses of the vaccine in Europe, and of course in Italy, by autumn-winter,” Ricciardi said.
The vaccine is being developed by Italian private company Advent-IRBM — based in Pomezia near Rome — and the Jenner Institute, which is part of the Oxford University in the United Kingdom. In April, the Anglo-Italian team announced the start of the human testing of the vaccine at the end of that month in the UK.
 A lab technician extracts portions of a COVID-19 vaccine candidate during testing at the Chula Vaccine Research Center, run by Chulalongkorn University in Bangkok. (AP)
A lab technician extracts portions of a COVID-19 vaccine candidate during testing at the Chula Vaccine Research Center, run by Chulalongkorn University in Bangkok. (AP)
💉 In another development in Europe that could help shots developed by companies like AstraZeneca and Johnson & Johnson, a Reuters report said European officials aim to speed up trials for coronavirus vaccines containing genetically modified organisms.
The strategy is aimed at securing enough doses of a possible vaccine for the bloc as it fears lagging behind the United States and China.The reform is expected to reduce member states’ power to impose extra requirements on drug companies when they conduct clinical trials on vaccines containing genetically modified organisms (GMOs).
“GMOs are very specific to very few vaccines based on adenovirus vectors,” Michel Stoffel of Vaccines Europe told Reuters, citing those developed by AstraZeneca and Johnson & Johnson among those that contain GMOs and would benefit from the possible changes.
Officials said as the Commission will also announce plans to use an emergency 2.4-billion-euro ($2.7 billion) fund to make advance purchases of promising vaccines against the novel coronavirus.
Another potential Covid-19 vaccine being developed by by Beijing Institute of Biological Products, which is affiliated to state-owned China National Pharmaceutical Group (Sinopharm), showed promise in trials in monkeys, triggering antibodies and raising no safety issues.
In a paper published by the medical journal “Cell”, researchers said the BBIBP-CorV vaccine induced high-level neutralising antibodies that can block the virus from infecting cells in monkeys, rats, guinea pigs and rabbits.
“These results support the further evaluation of BBIBP-CorV in a clinical trial,” researchers said in the paper.
Apart from BBIBP-CorV, Sinopharm, which has invested 1 billion yuan ($141.40 million) in vaccine projects, is testing in humans another vaccine candidate developed by its Wuhan-based unit. The two shots have been given to more than 2,000 people in clinical trials.


Comments are closed.